Articles
- Page Path
- HOME > J Trauma Inj > Volume 36(3); 2023 > Article
-
Review Article
Endovascular embolization of persistent liver injuries not responding to conservative management: a narrative review -
Simon Roh, MD

-
Journal of Trauma and Injury 2023;36(3):165-171.
DOI: https://doi.org/10.20408/jti.2023.0040
Published online: September 15, 2023
- 1,284 Views
- 60 Download
Department of Radiology, St. Luke’s Hospital, Temple University School of Medicine, Bethlehem, PA, USA
- Correspondence to Simon Roh, FINAL DEGREE Department of Radiology, St. Luke’s Hospital, Temple University School of Medicine, 801 Ostrum St, Bethlehem, PA 18951, USA Tel: +1-484-526-4805 Email: simon.roh@temple.edu
Copyright © 2023 The Korean Society of Traumatology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Trauma remains a significant healthcare burden, causing over five million yearly fatalities. Notably, the liver is a frequently injured solid organ in abdominal trauma, especially in patients under 40 years. It becomes even more critical given that uncontrolled hemorrhage linked to liver trauma can have mortality rates ranging from 10% to 50%. Liver injuries, mainly resulting from blunt trauma such as motor vehicle accidents, are traditionally classified using the American Association for the Surgery of Trauma grading scale. However, recent developments have introduced the World Society of Emergency Surgery classification, which considers the patient's physiological status. The diagnostic approach often involves multiphase computed tomography (CT). Still, newer methods like split-bolus single-pass CT and contrast-enhanced ultrasound (CEUS) aim to reduce radiation exposure. Concerning management, nonoperative strategies have emerged as the gold standard, especially for hemodynamically stable patients. Incorporating angiography with embolization has also been beneficial, with success rates reported between 80% and 97%. However, it is essential to identify the specific source of bleeding for effective embolization. Given the severity of liver trauma and its potential complications, innovations in diagnostic and therapeutic approaches have been pivotal. While CT remains a primary diagnostic tool, methods like CEUS offer safer alternatives. Moreover, nonoperative management, especially when combined with angiography and embolization, has demonstrated notable success. Still, the healthcare community must remain vigilant to complications and continuously seek improvements in trauma care.
- Incidence
- Trauma is a major burden on the healthcare system, with over five million fatalities per year worldwide [1]. A significant percentage of the patients involved in trauma are under the age of 40 years. The liver is a commonly injured solid organ in blunt and penetrating abdominal trauma [2,3], with an increased prevalence in recent decades [1,4–7]. Since uncontrolled bleeding can cause significant morbidity and has a mortality rate of 10% to 50% [8,9], it is important to control the hemorrhaging associated with liver trauma.
- A database analysis of trauma centers in the United States showed that patients with liver injuries had a mean age of 31.3 years and a mean Injury Severity Score of 23, and that 64% were male and 79% had sustained blunt injuries [2]. A retrospective analysis at a Norwegian trauma center showed that the median age of patients presenting with abdominal trauma was 31 years and that 70.4% were male. Adult patients comprised 83.3% of the patient population with 91.1% of the injuries due to blunt trauma [6].
- Causative factors
- Most liver injuries in the setting of trauma are due to blunt injuries. Blunt trauma most frequently results from motor vehicle crashes, followed by falls from a height and pedestrian versus automobile accidents [7,10].
- Classification
- Liver injuries have traditionally been classified according to the American Association for the Surgery of Trauma (AAST) organ injury grading scale (Table 1) [11]. A criticism of the AAST grading scale is the lack of correlation to the patient’s physiological status. A more recently devised classification system presented by the World Society of Emergency Surgery (WSES) does consider the patient’s physiological status (Table 2) [12].
- The WSES classifies liver injury according to three levels of severity. WSES grade I and AAST grades I–II include minor injuries, WSES grade II and AAST grade III include moderate injuries, and WSES grades III–IV and AAST grades IV–V include severe injuries. All AAST grades with hemodynamic instability are classified as severe [12].
INTRODUCTION
- Patients presenting to the trauma bay are evaluated for clinical stability by monitoring vital signs, assessing laboratory values, and performing a physical examination, after which a determination of hemodynamic stability can be made. The frequency of lab draws for hemoglobin assessment, the frequency of abdominal examinations, and the duration of monitoring vary depending on institutional protocols, and there are no official recommendations in terms of frequency and duration of monitoring [13]. Factors such as hemodynamic stability, the amount of acute blood loss, and the level of injury severity help determine whether patients can be managed nonoperatively [14].
CLINICAL EVALUATION
- Patients typically undergo multiphase computed tomography (CT) during the initial evaluation for liver injury as it can clearly show complications resulting from the trauma [15]. A significant number of traumatic injuries occur in young patients, and efforts to decrease radiation exposure during CT are important. Performing split-bolus single-pass CT during the evaluation for trauma has been shown to decrease radiation exposure for patients while maintaining or increasing image quality as compared to traditional multiphase CT [16]. Split-bolus single-pass CT also has the potential to decrease the turnaround time for CT reporting given the decreased number of images compared to multiphase CT.
- Contrast-enhanced ultrasound (CEUS) can also be useful in the evaluation of patients with liver injuries. CEUS has a higher sensitivity for detecting liver parenchymal lacerations than standard ultrasound [17]. The lack of ionizing radiation in CEUS exams also helps minimize patient exposure to radiation.
- In a comparison of patients with intraperitoneal and retroperitoneal hemorrhage, patients with retroperitoneal hemorrhage had greater injury severity. Liver injury was more common among patients with intraperitoneal hemorrhage than those with retroperitoneal bleeding [18].
DIAGNOSTIC EVALUATION
- Nonoperative management of liver trauma has become the gold standard [2,19] when treating patients who are hemodynamically stable, show low-grade liver injuries on imaging, have an absence of peritoneal signs, and require transfusion of less than 2 units of blood [20,21]. Temporary endovascular occlusion of the aorta for the control of severe intra-abdominal bleeding in trauma settings has been reported. Although no reports have described its use specifically in liver trauma [22], the traumatologist can consider its use in carefully selected cases.
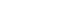
- According to the algorithm proposed by WSES, hemodynamically stable patients with grades I–III liver injuries can be managed nonoperatively. Patients that are hemodynamically unstable (WSES grade IV) or with imaging evidence of intraperitoneal free air and peritonitis are not candidates for nonoperative management. Patients that are treated nonoperatively and have imaging that shows signs of active contrast extravasation are candidates for angiography with embolization. Patients who undergo successful embolization can continue to be monitored nonoperatively, while those who do not need operative management [12,23].
- As stated in the WSES liver trauma management guidelines [23], there is growing evidence that nonoperative management is an option for patients with more severe liver injuries including AAST grades IV–V [21]. Inukai et al. [24] reported successful nonoperative management of patients with severe liver injuries (AAST grades IV–V) and found no significant difference in the development of biliary complications or abdominal compartment syndrome between hemodynamically stable and unstable patients.
- Although debate continues over which patients with liver injury should undergo angiography with embolization, Xu et al. [10] concluded that selective angiography in patients with AAST grades III–IV hepatic trauma resulted in a significant decrease in the failure of nonoperative management. Boonsinsukh and Maroongroge [25] demonstrated that patients with shock and hemodynamic instability following abdominopelvic trauma were successfully managed with arterial embolization. Tamura et al. [26] reported that patients with AAST grades III–V liver injuries and hemodynamic instability that responded to initial resuscitative efforts could be managed with embolotherapy without a significant difference in mortality or clinical failure compared to operative management. CT showing active contrast extravasation was shown to be an indication for hepatic arteriography with embolization regardless of the grade of liver injury [5]. Because the incorporation of angioembolization in the management of hepatic trauma patients varies, it is the traumatologist who must identify which patients will benefit from endovascular therapy.
- A study by Hwang et al. [27] found that, while most patients treated with embolization were treated nonoperatively, patients that underwent surgical management for trauma and developed persistent bleeding following surgery also had good outcomes with embolization. Hemodynamically unstable patients who respond to an initial fluid bolus are favorable candidates for nonoperative management or angiography with embolization [5,28]. Nonoperative management has shown a >90% success rate with decreased overall mortality, complications, and transfusion requirements compared to operative management [5]. A flow chart for the management of patients with liver trauma is presented in Fig. 1.
NONOPERATIVE MANAGEMENT
- With the increased involvement of interventional radiology in the management of patients with liver trauma, more hybrid rooms are being incorporated that combine an interventional radiology (IR) suite with a traditional operating room (OR) or emergency room (ER). One goal of a hybrid room is to facilitate interventions in patients requiring angiography for hepatic bleeding. The benefits of the hybrid room include reduced transport times and reduced mortality due to blood loss in patients with liver hemorrhage [29]. A hybrid room consisting of an emergency trauma bay and an IR suite was described by Ahn et al. [30] in the successful treatment of two renal trauma patients with severe injuries. This concept can also be applied to liver trauma settings without significant modification. A study showing the benefits of a hybrid OR demonstrated the ability to effectively control severe hemorrhage by combining the skillsets of IR and surgery [31]. Although that study also identified a prolonged time for transporting patients from the ER to the hybrid OR, the delay may have been partly due to the lack of a well-developed protocol for use of the hybrid OR.
HYBRID ROOM
- Selection of embolic material
- A wide variety of embolic materials exist for use in hepatic arteriography for trauma [32]. Embolic agents can be broadly classified into three categories: mechanical occlusion devices, particulates, and liquids and gels. Particulates can be further subclassified as permanent or temporary, and calibrated or noncalibrated. Liquids and gels can be further subclassified as sclerosing agents or gels, based on their properties (physical vs. chemical crosslinking) [32,33].
- Coils are the most widely used agents in hepatic trauma embolization [34]. Coils are composed of stainless steel, platinum, or nitinol and can be coated with fibers, proteins, or other bioactive materials to facilitate thrombus formation at the site of deployment. Coils can be deployed through catheters in a pushed, injected, or detached fashion, with the latter offering increased control at the site of embolization [35].
- Gelfoam is another embolic agent commonly utilized in hepatic trauma [34]. It is a temporary occlusive embolic agent composed of absorbable gelatin powder and falls into the category of noncalibrated particles [36]. The temporary nature aids in occlusion of hepatic arteries in the acute phase and allows recanalization of the occluded arteries once the patient has stabilized and no further bleeding is expected. Gelfoam has been associated with an increased risk for infection, which may hinder patient recovery postembolization [37].
- Polyvinyl alcohol (PVA) has also been used in liver trauma embolization [5]. PVA is categorized as a permanent particulate embolic agent. Although noncalibrated PVA exists, most PVA particles are used in the calibrated form with sizes varying from 100–300 µm to 900–1,200 µm [36]. PVA causes a mechanical occlusion of vessels and stimulates blood clot formation. Recanalization of the blood clot as well as particle migration may occur months after embolization [32].
- While liquid, gel, and particulate embolic agents are not typically used in the management of hepatic trauma embolization, N-butyl cyanoacrylate has been used in the management of trauma patients with arterioportal shunting or coagulopathy [26]. Liquid embolic agents can cause inadvertent distal embolization if reflux occurs. Particulate embolic agents can be considered in small distal arteries where there is minimal chance for reflux [38].
- Hepatic arterial selection
- Various types of base catheters can be used in selecting the celiac axis, including Sos, Cobra, or shepherd hook type catheters, depending on operator preference and the angle of celiac axis takeoff in relation to the aorta [39]. A review of preoperative CT scans may help to identify variant hepatic arterial branching anatomy. Microcatheters are advanced through the base catheter and used to selectively cannulate the right or left hepatic arteries, or further distally if needed. Virtual fluoroscopy combined with preoperative CT can aid in the selection of vessels [40].
- Selectivity of embolization
- Selective embolization of the hepatic arteries is important to minimize nontarget embolization and the consequences that can result from infarction of normal liver tissue [41]. Intraprocedural cone beam CT, which is frequently utilized in interventional oncology, may prove to be a useful tool in selecting hepatic arteries that may not be clearly visualized on standard digital subtraction angiography [42]. It is important to be as selective as possible in hepatic arterial embolization to minimize complications and to increase success rates for nonoperative management [5].
- Once the proper hepatic artery has been identified, expedited embolization of the artery is paramount in preventing blood loss. In coil embolization, oversizing of the coil by 20% to 30% is recommended to prevent migration [43]. The scaffold and anchoring techniques are also important to prevent migration and to provide efficient packing of the coils. The scaffold technique involves the initial placement of an oversized high radial force coil followed by a softer coil to maximize packing of the coils. The anchoring technique involves placing the initial coil into a branch vessel, which increases the stability of the initially placed coil, followed by an additional coil placement for complete embolization of the vessel [35].
EMBOLIZATION TECHNIQUES
- Success rates
- Embolization of hepatic injuries has been reported to be 80% to 97% successful [26]. The hepatic artery, portal vein, and hepatic vein are potential sources of bleeding in liver trauma. Identifying the exact source of bleeding can be difficult, especially when multiple vessels are injured, and can result in incomplete embolization with the need for further nonoperative or operative intervention [37]. Despite the potential limitations of embolization in patients with high-grade liver injuries (both operative and nonoperative), patients who underwent embolization had higher survival rates than those that did not [44].
- Complications
- Major complications arising after embolization for liver trauma included hepatic necrosis, hepatic ischemia, abscess, biloma, bile leak, gallbladder necrosis, pseudoaneurysm formation, and cholecystitis [5,9,10,26,28,45,46]. Sivrikoz et al. [44] reported a higher incidence of systemic complications following embolization including acute respiratory distress syndrome, sepsis, pneumonia, and renal failure.
- The liver receives its blood supply from the hepatic artery and portal vein. Disruption of both hepatic arterial and portal venous vasculature will result in hepatic ischemia and necrosis. Hepatic necrosis occurring after arterial embolization likely means there was concurrent portal vein disruption. Although one study showed hepatic necrosis rates up to 44% [46], most studies did not show rates nearly as high [9]. The low rates of hepatic necrosis after severe liver injury may be due to a compensatory increase in microcirculation that maintains liver perfusion despite the reduction in arterial and portal venous perfusion [47].
POSTEMBOLIZATION OUTCOMES
- Routine use of CT during the follow-up of liver trauma patients is not recommended, given the low rate of complications, especially in low-grade liver injuries [45]. CEUS may be useful in the follow-up of hemodynamically stable patients and also minimizes the use of ionizing radiation and iodinated intravenous contrast [17,48].
CONCLUSIONS
-
Conflicts of interest
The author has no conflicts of interest to declare.
-
Funding
The author did not receive any financial support for this study.
-
Data availability
Data of this study are available from the author upon reasonable request.
ARTICLE INFORMATION
AAST, American Association for the Surgery of Trauma.
Adapted from Kozar et al. [11], with permission from Wolters Kluwer Health Inc.
WSES, World Society of Emergency Surgery; AAST, American Association for the Surgery of Trauma.
Adapted from Coccolini et al. [12], available under the Creative Commons License.
- 1. Martin JG, Shah J, Robinson C, Dariushnia S. Evaluation and management of blunt solid organ trauma. Tech Vasc Interv Radiol 2017;20:230–6. ArticlePubMed
- 2. Tinkoff G, Esposito TJ, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg 2008;207:646–55. ArticlePubMedPMCPDF
- 3. Brillantino A, Iacobellis F, Festa P, et al. Non-operative management of blunt liver trauma: safety, efficacy and complications of a standardized treatment protocol. Bull Emerg Trauma 2019;7:49–54. ArticlePubMedPDF
- 4. Chaudhari PP, Rodean J, Spurrier RG, et al. Epidemiology and management of abdominal injuries in children. Acad Emerg Med 2022;29:944–53. ArticlePubMed
- 5. Virdis F, Reccia I, Di Saverio S, et al. Clinical outcomes of primary arterial embolization in severe hepatic trauma: a systematic review. Diagn Interv Imaging 2019;100:65–75. ArticlePubMed
- 6. Wiik Larsen J, Soreide K, Soreide JA, Tjosevik K, Kvaloy JT, Thorsen K. Epidemiology of abdominal trauma: an age- and sex-adjusted incidence analysis with mortality patterns. Injury 2022;53:3130–8. Article
- 7. Song IG, Lee JS, Jung SW, et al. Analysis of abdominal trauma patients using national emergency department information system. J Korean Soc Traumatol 2016;29:116–23. ArticlePubMed
- 8. Salazar GM, Walker TG. Evaluation and management of acute vascular trauma. Tech Vasc Interv Radiol 2009;12:102–16. ArticlePubMed
- 9. Samuels JM, Urban S, Peltz E, et al. A modern, multicenter evaluation of hepatic angioembolization: complications and readmissions persist. Am J Surg 2020;219:117–22. ArticlePubMedPMC
- 10. Xu H, Jie L, Kejian S, et al. Selective angiographic embolization of blunt hepatic trauma reduces failure rate of nonoperative therapy and incidence of post-traumatic complications. Med Sci Monit 2017;23:5522–33. ArticlePubMed
- 11. Kozar RA, Crandall M, Shanmuganathan K, et al. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg 2018;85:1119–22. ArticlePubMedPMCPDF
- 12. Coccolini F, Catena F, Moore EE, et al. WSES classification and guidelines for liver trauma. World J Emerg Surg 2016;11:50. PubMed
- 13. Stassen NA, Bhullar I, Cheng JD, et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73(5 Suppl 4):S288–93. ArticlePubMedPMC
- 14. Park KB, You DD, Hong TH, Heo JM, Won YS. Comparison between operative versus non-operative management of traumatic liver injury. Korean J Hepatobiliary Pancreat Surg 2015;19:103–8. ArticlePubMedPMCPDF
- 15. Yu SH, Park SH, Kim JW, et al. Imaging features and interventional treatment for liver injuries and their complications. Taehan Yongsang Uihakhoe Chi 2021;82:851–61. ArticlePubMedPDF
- 16. Jeavons C, Hacking C, Beenen LF, Gunn ML. A review of split-bolus single-pass CT in the assessment of trauma patients. Emerg Radiol 2018;25:367–74. PubMed
- 17. Catalano O, Lobianco R, Raso MM, Siani A. Blunt hepatic trauma: evaluation with contrast-enhanced sonography: sonographic findings and clinical application. J Ultrasound Med 2005;24:299–310.
- 18. Yoon JY, Kim SH, Ahn R, Hwang JC, Hong ES. Comparison of intraperitoneal and retroperitoneal/pelvic contrast extravasation: the characteristics and prognosis of the each patient group with arterial embolization according to the abdominal computed tomography scanning after blunt trauma. J Korean Soc Traumatol 2009;22:199–205. ArticlePubMed
- 19. Saqib Y. A systematic review of the safety and efficacy of non-operative management in patients with high grade liver injury. Surgeon 2020;18:165–77.
- 20. Yu HC. Nonoperative management guideline of liver injury. Korean J Hepatobiliary Pancreat Surg 2007;11:20–5. ArticlePubMedPMC
- 21. Brooks A, Reilly JJ, Hope C, Navarro A, Naess PA, Gaarder C. Evolution of non-operative management of liver trauma. Trauma Surg Acute Care Open 2020;5:e000551ArticlePubMed
- 22. Meyer DE, Mont MT, Harvin JA, Kao LS, Wade CE, Moore LJ. Catheter distances and balloon inflation volumes for the ER-REBOA™ catheter: a prospective analysis. Am J Surg 2020;219:140–4. ArticlePubMedPMCPDF
- 23. Coccolini F, Coimbra R, Ordonez C, et al. Liver trauma: WSES 2020 guidelines. World J Emerg Surg 2020;15:24. ArticlePubMedPDF
- 24. Inukai K, Uehara S, Furuta Y, Miura M. Nonoperative management of blunt liver injury in hemodynamically stable versus unstable patients: a retrospective study. Emerg Radiol 2018;25:647–52. ArticlePubMedPMC
- 25. Boonsinsukh T, Maroongroge P. Effectiveness of transcatheter arterial embolization for patients with shock from abdominopelvic trauma: a retrospective cohort study. Ann Med Surg (Lond) 2020;55:97–100. ArticlePubMedPMC
- 26. Tamura S, Maruhashi T, Kashimi F, et al. Transcatheter arterial embolization for severe blunt liver injury in hemodynamically unstable patients: a 15-year retrospective study. Scand J Trauma Resusc Emerg Med 2021;29:66. ArticlePubMedPMCPDF
- 27. Hwang MJ, Lee HK, Choi SJ, Chung SY. Clinical experiences of transarterial embolization after abdominal surgery in trauma patients. Korean J Vasc Endovasc Surg 2012;28:196–201. Article
- 28. Tan T, Luo Y, Hu J, Li F, Fu Y. Nonoperative management with angioembolization for blunt abdominal solid organ trauma in hemodynamically unstable patients: a systematic review and meta-analysis. Eur J Trauma Emerg Surg 2023;49:1751–61. Article
- 29. Prichayudh S, Rajruangrabin J, Sriussadaporn S, et al. Trauma Hybrid Operating Room (THOR) shortened procedure time in abdominopelvic trauma patients requiring surgery and interventional radiology procedures. Injury 2023;54:513–8. ArticlePubMed
- 30. Ahn SR, Seo SH, Lee JH, Park CY. Non-operative management with angioembolization of grade IV and V renal injuries in a hybrid emergency room system. J Trauma Inj 2021;34:191–7. ArticlePDF
- 31. Jang JY, Oh J, Shim H, et al. The need for a rapid transfer to a hybrid operating theatre: do we lose benefit with poor efficiency? Injury 2020;51:1987–93. ArticlePubMed
- 32. Hu J, Albadawi H, Chong BW, et al. Advances in biomaterials and technologies for vascular embolization. Adv Mater 2019;31:e1901071ArticlePubMedPMCPDF
- 33. Pal A, Blanzy J, Gomez KJ, Preul MC, Vernon BL. Liquid embolic agents for endovascular embolization: a review. Gels 2023;9:378. ArticlePubMedPMC
- 34. Bauer JR, Ray CE. Transcatheter arterial embolization in the trauma patient: a review. Semin Intervent Radiol 2004;21:11–22. ArticlePubMedPMC
- 35. Xiao N, Lewandowski RJ. Embolic agents: coils. Semin Intervent Radiol 2022;39:113–8. ArticlePubMedPMC
- 36. De la Garza-Ramos C, Salei A, Caridi TM. Nononcologic embolization. Semin Intervent Radiol 2022;39:416–20. ArticlePubMedPMC
- 37. Cadili A, Gates J. The role of angioembolization in hepatic trauma. Am Surg 2021;87:1793–801. ArticlePubMedPDF
- 38. O'Dell MC, Shah J, Martin JG, Kies D. Emergent endovascular treatment of penetrating trauma: solid organ and extremity. Tech Vasc Interv Radiol 2017;20:243–7. ArticlePubMed
- 39. Saiga A, Yokota H, Akutsu A, et al. Prediction of common hepatic artery catheter insertion based on celiac trunk morphology. Diagn Interv Radiol 2023;29:161–6. ArticlePubMedPMC
- 40. Tanaka Y, Oosone A, Tsuchiya A. Usefulness of virtual fluoroscopy in emergency interventional radiology. Taehan Yongsang Uihakhoe Chi 2020;81:852–62. ArticlePubMedPMCPDF
- 41. Pain JA, Heaton ND, Karani JB, Howard ER. Selective arterial embolization for hepatic trauma. Ann R Coll Surg Engl 1991;73:189–93. PubMedPMC
- 42. Lucatelli P, Corona M, Argiro R, et al. Impact of 3D rotational angiography on liver embolization procedures: review of technique and applications. Cardiovasc Intervent Radiol 2015;38:523–35. ArticlePubMedPDF
- 43. Lopera JE. Embolization in trauma: review of basic principles and techniques. Semin Intervent Radiol 2021;38:18–33. ArticlePubMedPMC
- 44. Sivrikoz E, Teixeira PG, Resnick S, Inaba K, Talving P, Demetriades D. Angiointervention: an independent predictor of survival in high-grade blunt liver injuries. Am J Surg 2015;209:742–6. ArticlePubMed
- 45. Virdis F, Podda M, Di Saverio S, et al. Clinical outcomes of non-operative management and clinical observation in non-angioembolised hepatic trauma: a systematic review of the literature. Chin J Traumatol 2022;25:257–63. ArticlePubMedPMC
- 46. Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma 2009;66:621–9. ArticlePubMed
- 47. Wong YC, Wang LJ, Wu CH, et al. Differences of liver CT perfusion of blunt trauma treated with therapeutic embolization and observation management. Sci Rep 2020;10:19612. ArticlePubMedPMCPDF
- 48. Di Serafino M, Iacobellis F, Schilliro ML, et al. The technique and advantages of contrast-enhanced ultrasound in the diagnosis and follow-up of traumatic abdomen solid organ injuries. Diagnostics (Basel) 2022;12:435. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 KST
KST

 PubReader
PubReader ePub Link
ePub Link Cite
Cite