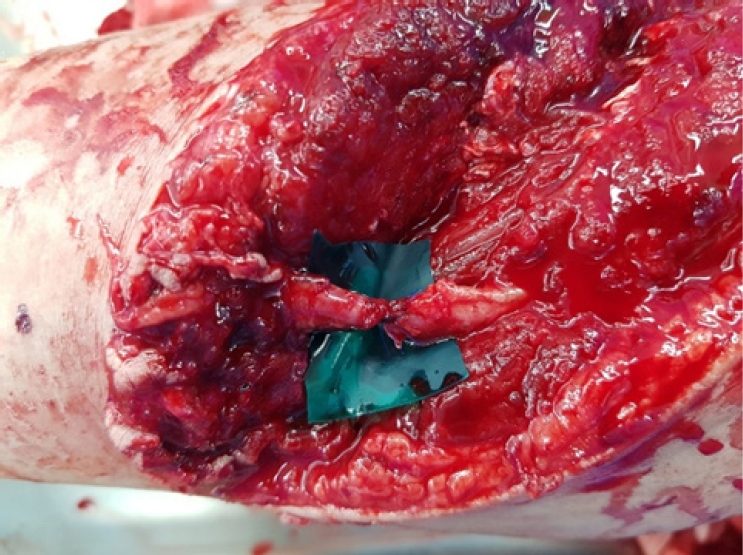
Experience of vascular injuries at a military hospital in Korea
Article information
Abstract
Purpose
Vascular injuries require immediate surgical treatment with standard vascular techniques. We aimed to identify pitfalls in vascular surgery for trauma team optimization and to suggest recommendations for trauma and vascular surgeons.
Methods
We reviewed 28 victims and analyzed the patterns of injuries, methods of repair, and outcomes.
Results
Ten patients had torso injuries, among whom three thoracic aorta injuries were repaired with thoracic endovascular aortic repair, one left hepatic artery pseudoaneurysm with embolization, and two inferior vena cava with venorrhaphy, three iliac arteries with patch angioplasty or embolization, and three common femoral arteries with bypass surgery or arterioplasty. Four patients had upper extremity injuries, among whom one brachial artery and vein was repaired with bypass surgery after temporary intravascular shunt perfusion, two radial arteries were repaired with anastomoses, and one ulnar artery was repaired with ligation. One radial artery under tension was occluded. Fourteen patients had lower extremity injuries, among whom one superficial femoral artery and vein was repaired with bypass and concomitant ligation of the deep femoral artery and vein, three superficial femoral arteries were repaired with bypass (two concomitant femoral veins with bypass or anastomosis), one deep femoral artery with embolization, two popliteal arteries with bypass or anastomosis, four infrapopliteal transected arteries, one arteriovenous fistula with ligation, and one pseudoaneurysm with bypass. However, one superficial femoral artery and all femoral veins were occluded. One leg replantation failed.
Conclusions
There are potential complications of vascular access during resuscitative endovascular balloon occlusion of the aorta procedures. Vascular repair should be performed without tension or spasm. Preservation of the harvested vein in papaverine solution and blood while using a temporary intravascular shunt is a method of eliminating spasms.
INTRODUCTION
Vascular trauma is a leading cause of death and disability in young adults, significantly affecting their future quality of life. It requires rapid diagnosis and immediate surgical treatment. It is often a part of polytrauma, requiring the multidisciplinary trauma team to perform various operations simultaneously. Most vascular injuries are managed by trauma surgeons with training or experience in vascular repair or ligation. Various options for vascular repair are available for a broad range of complex injuries [1]. The major pitfall when dealing with complex injuries is to assume that there will always be a skillful vascular surgeon to deal with all the issues involved [2]. We aimed to investigate the pitfalls and jobs of the vascular surgeon as a part of multidisciplinary team approach for trauma team optimization.
METHODS
We retrospectively reviewed the medical records of 28 patients discharged from a tertiary military hospital after surgical procedures for vascular injuries from November 1999 to October 2021. The reviewed data included trauma mechanisms, location and type of injured vessels, methods of repair, concomitant injuries, outcomes, and morbidity. Our evaluation focused on the methods of vascular repair and outcomes. This study was approved by the Institutional Review Board of Armed Forces Capital Hospital (No. 2002-03-004-002) and conducted in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
RESULTS
We identified 28 patients (27 men; mean age, 26.6±9.3 years; range, 19–59 years). Of these, eight (28.6%) presented with blunt injuries, 21 (75.0%) with penetrating injuries (including eight iatrogenic injuries, 28.6%). One blunt injury had a common femoral artery (CFA) dissection during a resuscitative endovascular balloon occlusion of the aorta (REBOA) procedure.
Five patients had thoracoabdominal vessel injuries (Table 1). In the three descending thoracic aorta (TA) injuries caused by a deceleration mechanism from a fall, thoracic endovascular aortic repair (TEVAR) was performed successfully without paraplegia. One had a concomitant left hepatic artery occlusion. It developed into a pseudoaneurysm and underwent coil embolization 34 days after the trauma (Fig. 1). Two inferior vena cava (IVC) injuries underwent venorrhaphy. One showed intramural hematoma at the infrahepatic IVC. It remained as an impending rupture. Another focal tear of the infrarenal IVC wall during discectomy caused hypovolemic shock. During repositioning to the supine position for exploratory laparotomy, the patient became pulseless and went into cardiac arrest. After stabilization with immediate resuscitative thoracotomy (RT) and aortic clamping, the wall defect was closed. Postoperatively, he showed symptoms of partial paraplegia.

Images of case 3. Pseudoaneurysm of the left hepatic artery (A) before and (B) after coil embolization on 34 days after trauma.
Five patients had iliofemoral artery injuries (Table 1). One wall defect (hemorrhage) of the common iliac artery (CIA) during L4/5 discectomy was repaired with patch angioplasty. Of two internal iliac artery (IIA) injuries, one transection of the distal branches by a gunshot was controlled with embolization. Another blunt injury from a fall showed transection of branches of the inferior gluteal artery with concomitant multiple lung contusions and hemorrhage, as well as multiple long bone and pelvis fractures. After a REBOA procedure, bleeding was controlled with embolization. A dissection of the CFA wall following blind removal of REBOA sheath occurred and was repaired with arterioplasty. However, the patient died of multiple organ dysfunction syndrome on day 44 of hospitalization. Two cases of intimal dissection with thrombotic occlusion at the CFA (iliofemoral junction) by an angio-access puncture for angiography or blunt trauma were repaired with extended polytetrafluoroethylene (ePTFE) iliofemoral bypasses.
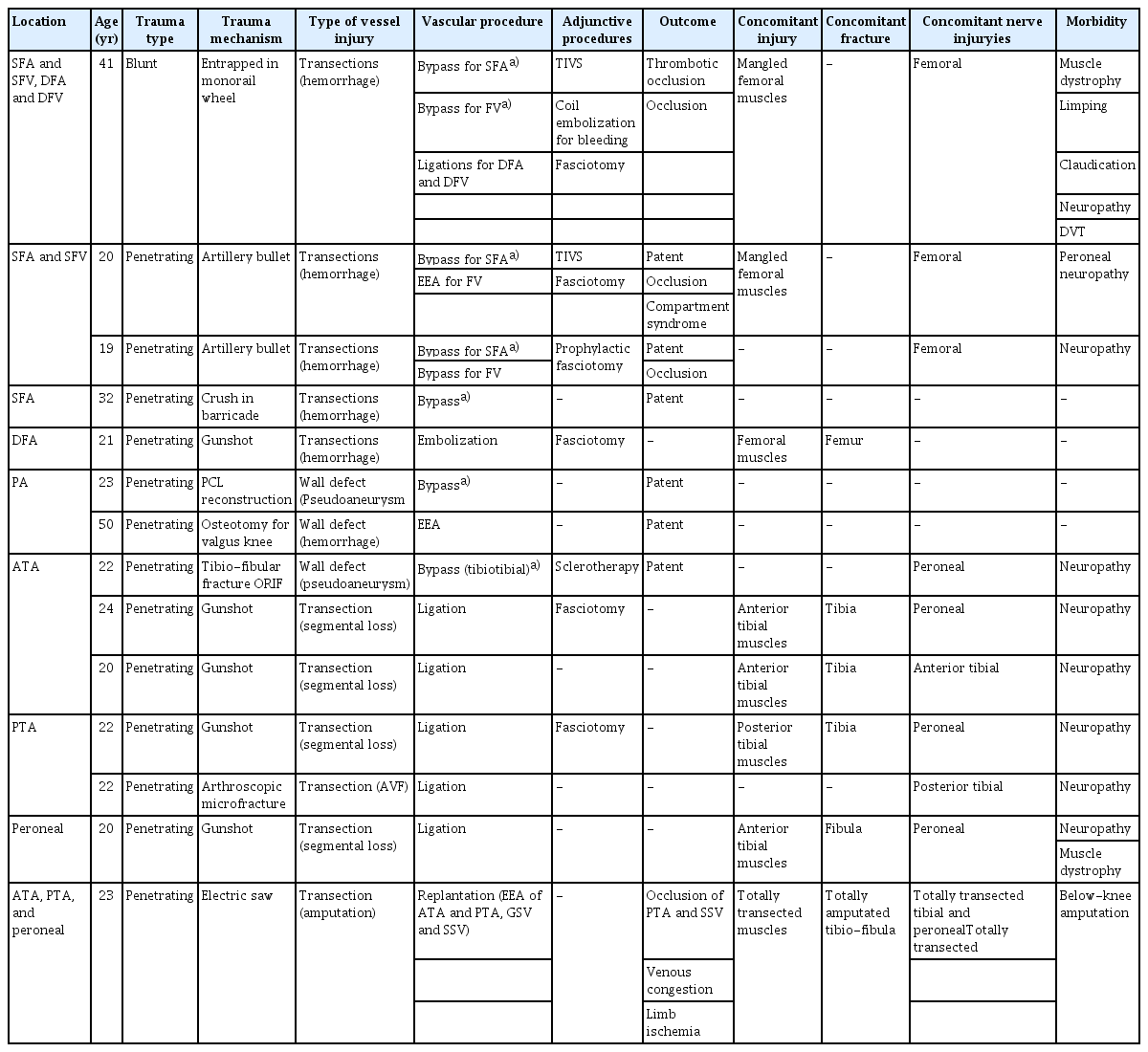
Four patients had vessel injuries in the upper extremity (Table 2). One transection (segmental loss) of the brachial artery (BA) and its accompanying brachial veins (BVs) by crushing was repaired with great saphenous vein (GSV) interposition bypass with good patency. Temporary intravascular shunt (TIVS) perfusion was performed during bone fixation and neurorrhaphy. Another crushing injury showed discontinuity of the radial artery (RA) and ulnar artery (UA) at the distal forearm on computed tomography angiography (CTA). The transected RA at 5 cm proximal to the wrist joint was repaired with end-to-end anastomosis (EEA). The UA was contracted by a severe spasm. Follow-up conventional angiography on the 7th postoperative day showed adequate distal flow to the palmar arch; however, spasms remained at the UA. The UA was finally occluded (Fig. 2). One RA transected by an electric saw was repaired with EEA under tension. Follow-up angiography on the 7th postoperative day showed occlusion (Fig. 3). Another gunshot injury showing 4-cm segmental loss of the UA underwent ligation without distal circulatory disturbance.
Images of case 12. (A) Computed tomography angiography showing discontinuity of radial and ulnar arteries at distal forearm. (B) The follow-up conventional angiography on 7th postoperative day showing adequate distal flow to palmar arch, but still remained spasm at ulnar artery.
Five patients had femoral vessel injuries (Table 3). One blunt injury caused by entrapment in a monorail wheel included transection of the superficial femoral artery (SFA) and femoral vein (FV), proximal deep femoral artery (DFA) and deep femoral vein (DFV), and mangled femoral muscle tears. Ligation of the proximal DFA and DFV showing segmental loss was performed. The SFA and FV were repaired with GSV interposition bypass. After referral to forward facilities, the FV became occluded with thrombosis during repeated debridement of nonviable muscles and wound irrigation. The GSV graft for SFA repair led to segmental thrombotic occlusion. Thrombectomy was tried 30 days after bypass surgery. A leakage occurred at the distal anastomosis site during thrombectomy, but the bleeding was controlled with coil embolization. Six months after embolization, CTA showed abundant collateral circulations without distal limb flow disturbance despite the occluded SFA and DFA (Fig. 4). The other three SFA transections caused by artillery shells or a barricade were repaired with GSV interposition bypass successfully. Another isolated transection of DFA by gunshot was controlled with embolization. Two concomitant FV transections were repaired with GSV interposition bypass or EEA. However, the FVs were occluded with thrombosis during repeated debridement.
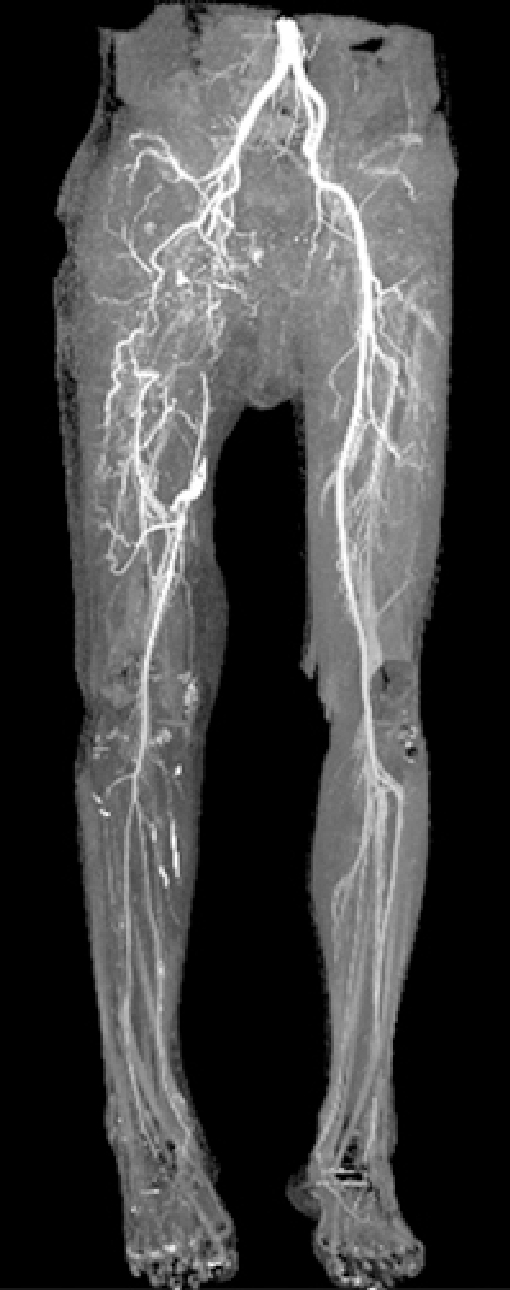
Case 15. Six months after embolization of superficial femoral artery. Computed tomography angiography showing abundant collateral circulations without distal limb flow disturbance despite occluded right superficial and deep femoral artery.
Two patients had popliteal artery (PA) injuries (Table 3). One pseudoaneurysm after posterior cruciate ligament reconstruction was repaired with GSV interposition bypass on the 21st postoperative day and a wall defect ongoing osteotomy for the valgus knee (distal femur) was repaired with EEA.
Seven patients had infrapopliteal vessel injuries (Table 3). One pseudoaneurysm of the distal anterior tibial artery (ATA) was diagnosed 2 months after open reduction and internal fixation of the fracture. Sclerotherapy using a thrombin injection failed, and GSV interposition bypass was performed. Another arteriovenous fistula (AVF) of the posterior tibial artery (PTA) after arthroscopic microfracture of the talus was ligated 2 weeks after injury. Four segmental defects at the ATAs, PTA, and peroneal artery by gunshot were ligated. One had a right leg amputated at 5 cm above the tibiotalar joint by an electric saw. The emergency dispatch team wrapped the amputated limb in saline-soaked gauze and kept it in a cooled ice box for 2 hours. After bone shortening (4 cm) and fixation, EEA for foot replantation was performed in the order of PTA, GSV, ATA, and the small saphenous vein (SSV). The cold ischemia time was 6 hours. Finally, the dominant ATA and GSV became patent. Marked venous congestion with foot muscle ischemic changes led to the loss of foot viability, and the patient underwent below-knee amputation.
DISCUSSION
Mechanisms of injury are divided into blunt or penetrating. In recent years, with the development of mini-invasive and endovascular techniques, there has been an increase in iatrogenic vascular injuries. Most civilian casualties occur by penetrating objects such as firearms, bullets, stabbing, and machinery resulting from criminal acts, as well as traffic and labor accidents [1]. Most stab or gunshot wounds cause limited tissue damage or simple fractures that demand minimal wound debridement and bone fixation. Vascular injuries can be treated without considering the damage in other tissues [3]. Most cases of vascular trauma in the military occur from fragments from ballistic weaponry [4]. High-velocity weapons cause more massive tissue destruction or compound fractures. These injuries require early restitution of blood flow of the large, damaged vessels by a shunt while time is consumed by initial wound debridement and fracture stabilization until definitive vascular repair can be achieved [3].
Hemodynamically unstable patients may require the employment of damage control approaches such as REBOA or RT and aortic clamping or TIVS. REBOA confers significant risks associated with arterial access and ischemia-reperfusion. REBOA-related vascular injuries are exaggerated because many interventions are performed by surgeons who have limited experience in endovascular techniques, inadequate resources, and minimal training in the technique [5,6]. A CFA tear following REBOA sheath removal may require various vascular procedures, including thrombectomy, dissection flap repair, patch angioplasty, interposition grafting, or bypass [7]. We experienced a case of massive bleeding caused by CFA dissection following blind REBOA sheath removal, which was repaired with arterioplasty. All REBOA procedures should be performed under fluoroscopic guidance in a hybrid fashion to optimize patient outcomes. Another option is RT and descending aorta clamping as a last-resort procedure to resuscitate a patient who has gone into cardiac arrest. The distal organs are at risk of paraplegia-associated ischemia due to cross-clamping of the aorta [8]. We experienced a case of paraplegia. The trauma teams should be aware of the potential contribution of systemic hypotension to spinal cord ischemia following aortic occlusion. TIVS is a useful first step for limb salvage in a patient with a mangled extremity or near exsanguination [9]. When placed intraarterially, shunts facilitate perfusion until definitive repair is possible. For venous injuries, a temporary shunt provides drainage and decreases venous hypertension. TIVS was used at our facility as a temporizing adjunct during mass casualty situations, completion of bony fixation, and during concomitant venous repair or preservation of a harvested vein graft.
The five recognized types of vascular injuries are intimal injuries (flaps, disruptions, or subintimal/intramural hematomas), complete wall defects with pseudoaneurysms or hemorrhage, complete transections with hemorrhage or occlusion, AVF, and spasm [10]. Intimal defects and subintimal hematomas with possible secondary occlusion are most commonly associated with blunt trauma, while wall defects, complete transections, and AVF usually occur with penetrating trauma [4,10]. A laceration encompassing greater than 25% of the circumference of the artery increases the risk of distal embolization of a local clot [9].
A vascular spasm is a challenging problem to resolve. A peripheral artery spasm in an injured extremity is a common imaging finding in young patients. However, there remains much debate over the existence of trauma-induced arterial spasms in the absence of arterial wall damage. It may arise as a result of external mechanical stimulation caused by bone fragments or transmitted shockwaves [10]. If distal flow to the hand or foot is intact, observation is appropriate [10]. The restoration of a normal hemodynamic state, reversal of hypothermia, and topical warming of the injured extremity will reverse arterial spasms in 6 to 8 hours in most patients. Another treatment option for spasms in small or medium-sized arteries is an intraarterial bolus injection of papaverine or tolazoline, or nitroglycerin in angiography suites and nifedipine (per os or sublingual) [9].
Arterial surgery implies arterial dissection. A regular sequela of arterial dissection is a traumatic arterial spasm. It is more frequent and more severe in smaller vessels. Spasm makes suture placement difficult. Vascular spasm after bypass surgery causes decreased distal blood flow, increases the risk of thrombus formation, and can lead to functional damage distally. The recommended intraoperative options to prevent vessel spasms include rapid rewarming with warm saline (>37 °C), the use of a small hemoclip or bipolar cauterization, stretching the adventitia, or cutting the adventitia at the point of narrowing [11]. The application of pharmacological vasodilators is a common practice, as it is believed to reduce vasospasm and dilate vessels. The commonest preparations for topical use are phosphodiesterase inhibitors (e.g., papaverine), calcium channel blockers (e.g., verapamil), local anesthetics (e.g., lidocaine), and direct vasodilators (e.g., sodium nitroprusside) [11,12]. For instance, intraluminal, topical, and even perivascular infiltration are individually optimal methods for administering papaverine. Gentle mechanical dilation with vascular dilators or hydrostatic pressure might be useful for eliminating spasms. Spam reversal could result in an easier and more accurate anastomosis and possibly improved patency rates following sutures [12]. We routinely preserve the harvested GSV segment in a warm solution of papaverine mixed with the patient’s own blood until the complete relief of spasm during the fixation of a fractured bone and nerve repair.
A definitive vascular repair should be performed in situations with more normalized physiology. The recommended options for peripheral vascular repair are arterioplasty or venorrhaphy, patch angioplasty, resection with EEA, resection with interposition bypass, extraanatomic bypass, and ligation [13]. In damage control settings, ligation may be considered for specific vessels. It must be recognized that the principle of “life over limb” applies in specific settings [7]. Generally, all major named arteries should be repaired or reconstructed if possible [5]. Lateral repair is possible if there is a lateral tear only. Patch angioplasty with a vein or synthetic graft is not a common repair technique in penetrating trauma; however, it can be used for larger lacerations [3]. The adjunctive utilization of vascular pledgets is useful in specific large vessel repairs, particularly for thin-walled venous structures such as the IVC [5]. Primary repair is only possible in directly cut vessels where the clean vessel stumps can come together in a tension-free fashion after mobilization from the surrounding tissue [5]. An EEA performed under tension will result in an ''hourglass'' narrowing that increases the risk of early postoperative thrombosis [2,9]. We experienced a case of RA thrombotic occlusion that was repaired with an EEA under tension. If a segmental loss of >2 cm is present, the GSV should be harvested from the contralateral limb as the best choice of a graft. These native vessels have a lower risk for potential infection [5]. If the GSV is unavailable, the SSV or basilic or radio-cephalic vein or synthetic prosthesis should be considered [1,10]. PTFE has a satisfactory patency when grafts with a diameter greater >6 mm are used. It is remarkably resistant to infection in the absence of exposure or adjacent osteomyelitis [9,10]. PTFE may be used in a contaminated field. An effort should be made to obtain soft tissue coverage [14].
Wide debridement of wound contamination and nonviable tissue should be undertaken. Vascular repairs should be covered with viable tissues, or else infection, desiccation, and obstruction during repeated postoperative serial debridement and irrigation of mangled infected soft tissue can occur. Exposure of a lateral repair, EEA, or saphenous vein graft will result in contamination, leading to infection [9]. We experienced occlusions of one SFA and three FVs, which were repaired with either GSV interposition or EEA during repeated postoperative serial debridement of mangled, infected soft tissue. We reviewed a case of anastomosis leakage during thrombectomy of GSV graft obstruction for SFA repair 30 days after bypass surgery. The bleeding was controlled with coil embolization. We recommend in situ redo bypass surgery or extraanatomic bypass instead of an interventional trial for a thrombosed graft.
Blunt TA injury of an intimal tear, wall defect, or pseudoaneurysm is usually an effect of road traffic accidents or a fall resulting from acceleration-deceleration mechanisms. Most blunt aortic injuries occur at the aortic isthmus [1,15]. The damage requires immediate surgical exploration, and open repair (primary closure, patch angioplasty, or an interposition graft with ePTFE) usually requires high aortic clamping. However, the risk of a neurological deficit resulting from spinal cord ischemia is significantly higher in open repair than in TEVAR. The TEVAR technique has significantly improved survival and replaced open surgery. Endograft malposition and spinal ischemia are the most important potential acute complications of left subclavian artery coverage [5,15]. We reviewed three cases of TA injuries that underwent TEVAR successfully without paraplegia.
Abdominal vascular injuries cannot be temporarily controlled by external pressure [5]. A penetrating injury of the IVC usually produces a large retroperitoneal hematoma and tends to self-cease with the drop in the blood pressure and compression produced by the hematoma. However, this condition is unstable, and the patient may die suddenly from irreversible hypovolemic shock. Direct sponge stick compression above and below the IVC injury is effective for temporary bleeding control [5]. Injuries encompassing <50% of the vessel wall and those that can be closed transversely should be repaired with lateral venorrhaphy. In defects of >50% of the vessel circumference, primary repair results in significant narrowing [16]. We repaired two IVC injuries with venorrhaphy. One patient experienced a sudden drop in blood pressure during repositioning for exploratory laparotomy. Immediate RT and aortic clamping saved the patient from cardiac arrest. The trauma team should remember that IVC injuries not tamponaded by hematoma are prone to unresponsive hypovolemic shock during transport and even position change.
Posttraumatic hepatic artery pseudoaneurysm is uncommon. Most cases (80%) are extrahepatic and have a late onset [17]. Delayed complications can occur from weeks to months after the trauma. Although they are usually asymptomatic, they should always be treated because of the high risk of rupture. Currently, the treatment of choice is endovascular coil embolization [18]. We treated a pseudoaneurysm with coil embolization on the 34th day after injury.
Blunt iliac artery injuries usually result from pelvic polytrauma with multiple pelvis fractures. In most cases, a multidisciplinary approach of a specialized trauma team is necessary. The vascular surgeon’s job is to stop the bleeding and to perform vascular reconstruction [1]. Embolization or vessel ligation is the most common solution in an IIA injury, and reconstruction with prosthetic grafts is the first choice in a CIA or external iliac artery injury. Venous grafting has limited applicability owing to the insufficient diameters of the available veins [1].
Penetrating external iliac artery injuries, especially in the inguinal ligament region (death-triangle injuries) need a rapid surgical decision to save the patient’s life. Bleeding control in that region is difficult and possible only by direct compression. The open repair method is the gold standard. For the proximal CFA, in a highly flexible ilioinguinal area, the vein diameter may not be adequate. A synthetic graft is an acceptable alternative [3,5]. We successfully repaired iliac artery injuries with angioplasty or iliofemoral bypass with an ePTFE graft.
The BA allows its own mobilization with dissection and exposure, facilitating an EEA in many cases [5]. In a damage control situation, a TIVS can be placed quickly. Small lacerations are repaired with interrupted sutures. A more extensive injury is repaired with EEA, or interposition vein grafting [3,5]. We repaired one segmental BA and BV loss with spasm-free GSV interposition grafts.
Single arterial injury, isolated AVF, and pseudoaneurysm in the forearm can be treated by ligation or embolization, provided palmar arch patency is preserved [5]. However, repair is required if both the RA and UA are injured or when there is an incomplete palmar arch. The UA should preferentially be repaired because it is usually the dominating vessel. We repaired the two isolated, transected RA with EEA; however, one anastomosis under tension led to thrombotic occlusion.
The inguinal ligament can be divided if additional proximal femoral artery exposure is required. The SFA is commonly repaired with a short-segment interposition of an ePTFE graft or via vein patching. All proximal DFA injuries should be optimally repaired with a short interposition graft or reimplantation to the SFA. Ligation of the proximal DFA is only acceptable in the extremes of damage control for unstable patients with multiple injuries. The SFA is best repaired with a GSV interposition graft. These injuries are rarely amenable to EEA or patch repair [5]. We repaired four transected SFA with GSV interposition grafts. One thrombotic occlusion led to rupture during thrombectomy, and coil embolization was performed. Even with concomitant ligation of the DFA, the abundant collateral circulations to distal flow saved the patient from amputation.
Most PA injuries can be repaired with either an EEA or a GSV interposition graft. Associated venous injuries should be treated with primary or patch repair if possible. Prophylactic, four-compartment lower extremity fasciotomy should be strongly considered for occlusive popliteal injuries with a prolonged warm ischemic time [5]. We successfully repaired one PA pseudoaneurysm with a GSV interposition graft and one wall defect with EEA.
Injury to the tibioperoneal trunk usually requires vascular repair [3]. The surgical repair of an isolated single-vessel tibial artery injury below the tibioperoneal trunk injury is not indicated. At least one tibial artery should be repaired for patients with multiple injuries. Bypass repair with an interposition GSV graft is usually required [5]. Extravasation or pseudoaneurysm can be treated with open ligation or with embolization [10]. We experienced seven infrapopliteal vessel injuries; we ligated segmental defects or AVFs and repaired pseudoaneurysms with a vein graft. We attempted one foot replantation; however, it was unsuccessful. The main cause of failure was improper management during the cold ischemic time. Hence, we suggest that the trauma team should prepare an organ preservation protocol for prolonged cold ischemia.
There has been an ongoing discussion on how to best manage extremity venous injuries. In patients with life-threatening injuries, the ligation of extremity venous injuries is still recommended. Vein repair was recently recommended for isolated extremity injuries or vascular injuries with other non-life-threatening injuries [19]. Minor venous injuries are repaired with a lateral suture technique; however, luminal narrowing should not be more than 50%. Vein patch angioplasty should be considered for larger lacerations. An interposition graft is required for segmental loss. An autogenous vein is used if the size matches and if there is no contamination and good tissue coverage. The long-term patency of PTFE can definitively be questioned, although it can serve as a bridge to improved arterial patency and minimized postoperative swelling [19]. Panel or spiral venous grafts have been suggested as interposition grafts in the more distal FV, common FV, or axillary vein [2]. Injuries to the FV and the popliteal vein should preferably be repaired [19]. An aggressive approach toward extremity venous injury repair seems justified because of its likely role in reducing venous hypertensive sequelae as well as a potential role in limb salvage [19]. We experienced three cases of FV reconstruction with EEA or vein interposition grafts occluded in mangled injuries with simultaneous infection. Maintaining the patency of lower extremity vein reconstruction laid open in an infected wound is a challenging problem.
A limitation of this study is that the victims were not treated at a single center and by a single team. Some patients were administered first aid or surgery at nearby trauma units at the scene, and some were transported to forward facilities after primary care.
There are potentially catastrophic complications of vascular access or associated ischemia to distal organs during REBOA or RT procedures. Bleeding not tamponaded by the retroperitoneum can lead to irreversible hypovolemic shock during transport or even position change in an IVC injury. TIVS is an important adjunct to definitive repair in patients with a mangled extremity. It should be used liberally as the first step in limb salvage to restore arterial inflow and venous outflow. Vascular repair should be performed by trained vascular or trauma surgeons following basic principles. Vascular repairs should be done without tension or spasm. The preservation of the harvested vein in a solution of papaverine mixed with the patient’s own blood is a method of eliminating spasms. Maintaining the patency of FV reconstruction in a mangled injury is challenging. The pitfalls learned from this review will provide useful recommendations for constantly rotating trauma/vascular surgeons, since they are a part of multidisciplinary team approach for trauma team optimization.
Notes
Ethics statements
This study was approved by the Institutional Review Board of Armed Forces Capital Hospital (No. 2002-03-004-002) and conducted in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
None.
Author contributions
Conceptualization: HCK; Data curation: DK, SN, YHL, HL; Formal analysis: DK; Methodology: HCK; Project administration: HCK; Visualization: SN, YHL, HL; Writing–original draft: DK, HCK; Writing–review & editing: all authors. All authors read and approved the final manuscript.