Articles
- Page Path
- HOME > J Trauma Inj > Volume 32(4); 2019 > Article
-
Original Article
Outcomes of Cranioplasty Using Autologous Bone or 3D-Customized Titanium Mesh Following Decompressive Craniectomy for Traumatic Brain Injury: Differences in Complications - Junwon Kim, M.D.1, Jang Hun Kim, M.D.1,2, Jong Hyun Kim, M.D., Ph.D.1, Taek-Hyun Kwon, M.D., Ph.D.1, Haewon Roh, M.D.1,3
-
Journal of Trauma and Injury 2019;32(4):202-209.
DOI: https://doi.org/10.20408/jti.2019.033
Published online: December 30, 2019
- 5,362 Views
- 98 Download
- 6 Crossref
1Department of Neurosurgery, Guro Hospital, Korea University College of Medicine, Seoul, Korea
2Trauma Center, Armed Forces Capital Hospital, Seongnam, Korea
3Focused Training Center for Trauma, Guro Hospital, Korea University College of Medicine, Seoul, Korea
- Correspondence to: Haewon Roh, M.D., Department of Neurosurgery and Focused Training Center for Trauma, Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-ru, Seoul 08308, Korea, Tel: +82-2-2626-1178, Fax: +82-2-863-1684, E-mail: rofree1st@gmaillcom
• Received: September 10, 2019 • Revised: October 25, 2019 • Accepted: November 21, 2019
Copyright © 2019 The Korean Society of Trauma
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Purpose
- Cranioplasty (CP) is often required for survival after decompressive craniectomy. Several materials, including autologous bone and various artificial materials, have been introduced for CP, but it remains unclear which material is best for CP. This study aimed to explore differences in complications between patients who underwent CP using an autologous bone flap versus a three-dimensional (3D) titanium mesh and to identify significant risk factors for post-CP complications.
-
Methods
- In total, 44 patients were enrolled in this study and divided into two groups (autologous bone vs. 3D titanium mesh). In both groups, various post-CP complications were evaluated. Through a comparative analysis, we aimed to identify differences in complications between the two groups and, using binary logistic analysis, to determine significant factors associated with complications after CP.
-
Results
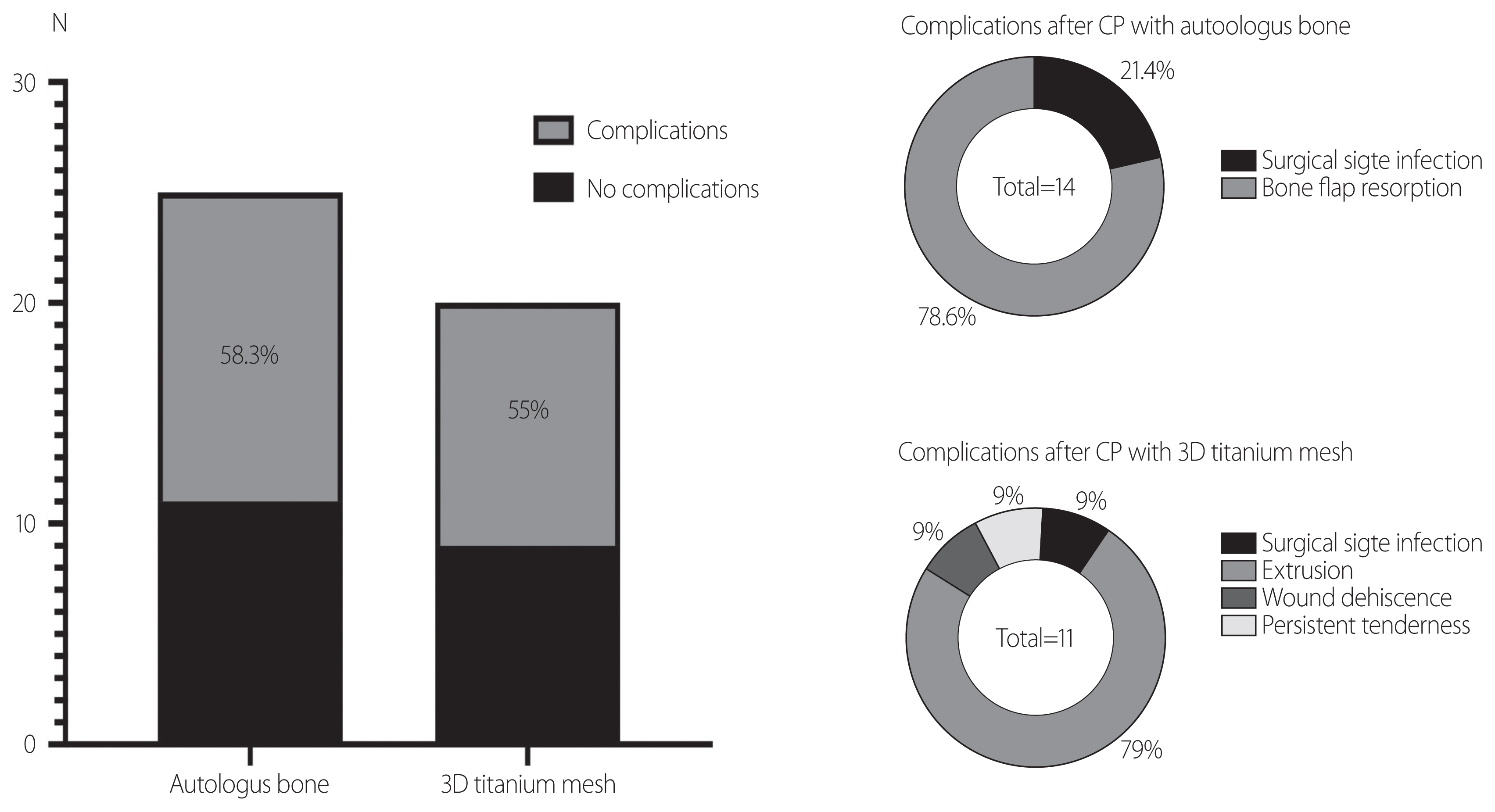
- In the autologous bone flap group, there were three cases of surgical infection (3/24, 12.5%) and 11 cases of bone flap resorption (BFR) (11/24, 45.83%). In the 3D titanium mesh group, there was only one case of surgical infection (1/20, 5%) and 11 cases of various complications, including mainly cosmetic issues (11/20, 55%). A subgroup risk factor analysis of CP with an autologous bone flap showed no risk factors that predicted BFR with statistical significance, although a marginal association was found between larger bone flaps and BFR (odds ratio [OR]=1.037, p=0.090). In patients treated with a 3D titanium mesh, multivariate analysis revealed that only the existence of a ventriculo-peritoneal shunt system was strongly associated with overall post-CP complications (OR=18.66, p=0.021).
-
Conclusions
- Depending on which material was used, different complications could occur, and the rate of complications was relatively high in both groups. Hence, the material selected for CP should be selected based on individual patients’ conditions.
- As some trials have demonstrated the efficacy of decompressive hemicraniectomy (DC) for severe traumatic brain injury (TBI) in terms of reducing mortality and improving functional outcomes, it has been widely used as salvage treatment for malignant edema of the brain after TBI [1,2]. To relieve and stabilize brain edema in patients who survive for a sufficient time, cranioplasty (CP) is required to restore the appearance of the skull, protect intracranial tissue, and improve neurological function [3,4].
- Several materials, including autologous bone and various artificial materials, have been introduced for CP, but which material is best for CP still remains unclear [5–7]. Among these materials, autologous bone flaps are usually the first choice at most institutions, as they are readily available at no additional cost and yield excellent outcomes [8,9]. Autologous bone also has the potential to grow and fuse with bone at the surgical site and is psychologically acceptable to patients and their relatives [10]. However, complications such as surgical site infection and bone flap resorption (BFR) always remain possible [8,9]. Various artificial materials (e.g., polymethyl methacrylate, titanium, ceramics, and carbon fiber-reinforced polymers) have been produced to reduce these bioactive complications [5,7,11]. Of these artificial materials, titanium mesh is generally considered the material of choice in moderate-sized to large craniectomy defects because it is biologically inert, strong, lightweight, and inexpensive to produce [4,12]. Furthermore, as advances in technology and imaging processes have enabled the production of large three-dimensional (3D) custom-made prefabricated plates, 3D customized titanium has been widely used [5]. However, several studies have reported that CP with titanium material is strongly associated with wound or cosmetic complications [3,4,6]. This study was conducted to explore differences in complications between patients who underwent CP using an autologous bone flap to those who underwent CP using a 3D titanium mesh. Furthermore, a subgroup analysis to identify risk factors for complications in each group was performed.
INTRODUCTION
- Patients, clinical variables, and definitions of various complications
- From January 2015 to December 2017, 44 patients (24 patients who underwent CP using an autologous bone flap and 20 patients who underwent CP using a 3D titanium mesh) were enrolled (Fig. 1). They had all previously been treated with DC for refractory increased intracranial pressure resulting from trauma. Clinical data of enrolled patients were collected from their medical charts, images, and telephone interviews with patients or their families. To more precisely identify the differences that resulted from the material used for CP, we excluded factors associated with surgical procedures, such as postoperative subdural hematoma, epidural hematoma, and subdural hygroma. Therefore, data on patients’ demographic characteristics and possible risk factors—including age, sex, operative time for CP, flap size (autologous bone flap size or titanium mesh size), existence of a ventriculo-peritoneal (V-P) shunt system, and the interval between DC and CP—were collected. In order to measure the area of autologous bone flaps and customized 3D titanium mesh plates, Image J software (http://imagej.nih.gov/ij/). Using that software, the surface area of the implanted bone flaps or implanted titanium mesh plates was measured by manually tracing their outlines on lateral skull plain images.
- Various complications after CP (e.g., postoperative surgical infections, BFR, wound dehiscence, and persistent pain after wound healing) were evaluated. Surgical infection was defined clinically and radiologically by any evidence of infection, including fever, erythema, swelling, and elevated levels of inflammatory markers with evidence of infection on computed tomography (CT) using contrast medium. BFR was diagnosed with serial brain CT. We defined BFR as more than 50% thinning of the bone flap compared to the thickness of the contralateral region (Fig. 2). Mesh extrusion was defined as severe thinning of the soft tissue and skin to show implanted mesh patterns without wound dehiscence (Fig. 3). Persistent tenderness at the surgical site was defined as severe pain caused by a gentle touch at the surgical site after the wound had totally healed without any dehiscence. Overall complications were defined as all kinds of complications after CP. We performed risk factor analysis both for overall complications in both groups and for specific complications (BFR and surgical site infection in the autologous bone flap group, and surgical site infection, extrusion, wound dehiscence, and persistent tenderness at the surgical site in the 3D titanium mesh group. To summarize, age, sex, operative time for CP, flap size (autologous bone flap size or titanium mesh size), the existence of a V-P shunt, and the interval between DC and CP were analyzed as independent variables and various complications (BFR, surgical infection, and other problems) after CP in both groups were analyzed as dependent variables. Institutional Review Board acceptance was obtained for this retrospective study and the need for informed consent was waived (IRB No. R2019-0055-001).
- DC and CP procedures
- We performed DC when patients showed clinical deterioration resulting from refractory increased intracranial pressure despite the best possible medical treatment. All patients underwent standard unilateral frontotemporal-parietal craniectomy with durotomy and duroplasty using artificial dura mater. The removed bone flap was separated from the adherent tissue, packed in sterile medical towels, and stored at −80°C immediately after surgery. The choice of material for CP depended on the surgeon’s preference and the interval between DC and CP. We used a 3D titanium mesh plate in all cases with more than a 3-month interval. Before performing CP, the patient’s hair was completely removed with an aseptic medical shaver. The scalp was then washed with an aqueous solution of chlorhexidine gluconate and left to dry, after which povidone-iodine solution was applied several times to the patient’s entire head and left to dry. The previous skin incision was re-opened, and the fibrous layer between the artificial dura and galea was dissected and prepared for insertion of the autologous bone or titanium mesh. After the bone flap was washed several times with povidone-iodine and normal saline solution, the flap was re-implanted with multiple tack-up sutures and fixed in its original position using miniature titanium plates and screws. In the final step, the skin was closed with vicryl subcutaneous sutures and nylon skin sutures with medical staples.
- Statistical analysis
- Data were presented as the mean and standard deviation for continuous variables and as the frequency or percentage for categorical variables. The analysis was carried out using the independent t-test and the Fisher’s exact test. Univariate and multivariate binary logistic regression analyses for complications were conducted, and all factors that showed significance in the univariate analysis were included in the multivariate logistic regression analysis. A p≤0.05 was considered to indicate statistical significance. All analyses in the present study were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
METHODS
- Patients’ demographic information and clinical details are presented in Table 1. The mean age of patients treated with CP using an autologous bone flap was 49.0±17.32 years (range, 16–75), while the mean age of patients who underwent CP using a 3D titanium mesh was 53.65±11.50 years (range, 27–81). The mean flap size was 104.17±27.10 cm2 in the autologous bone flap group and 122.56±34.8 cm2 in the 3D titanium mesh group. The mean operative time was 196.71±75.62 mintues in patients who underwent CP with an autologous bone flap and 170.37±128.30 mintues in patients who underwent CP with a 3D titanium mesh. The mean DC-CP interval was 66.29±42.23 days in the autologous bone flap group and 253.45±351.66 days in the 3D titanium mesh group. Shunt-dependent hydrocephalus occurred in 10 patients who underwent CP with an autologous bone flap (10/24, 41.7%) and in 8 patients who underwent CP with a 3D titanium mesh (8/20, 40%). The only statistically significant difference found between the groups was in the DC-CP interval (p=0.009).
- In the autologous bone flap group, there were three cases of surgical infection (3/24, 12.5%), 11 cases of BFR (11/24, 45.83%), and no other complications. In CP patients with a 3D titanium mesh, there was only one case of surgical infection (1/20, 5%), while 11 cases of various complications including extrusion, wound dehiscence, and persistent tenderness at the surgical site were noted (11/20, 55%) (Fig. 4).
- Overall complications were defined as including all kinds of complications after CP. We performed risk factor analysis both for overall complications in both groups and for specific complications (BFR and surgical site infection in the autologous bone flap group, and surgical site infection, extrusion, wound dehiscence, and persistent tenderness at the surgical site in the 3D titanium mesh group).
- In each subgroup analysis of risk factors for complications, we failed to find any significant risk factors for overall complications—including BFR and surgical infection—in patients treated with CP using an autologous bone flap, although the univariate binary logistic analysis yielded a marginal tendency for larger bone flaps to be related to BFR (Exp [B]=1.037, p=0.090, 95% confidence interval [CI]: 0.994–1.081). However, in patients treated with a 3D titanium mesh, multivariate analysis revealed that the only the existence of a V-P shunt system was strongly associated with overall complications, including surgical infection, extrusion, wound dehiscence, and persistent tenderness after CP (odds ratio=18.66, p=0.021, 95% CI: 1.56–222.92).
RESULTS
- Various complications after CP were found, and regardless of which material was used, the rate of complications was relatively high [3,4,12–14]. In patients treated with CP using an autologous bone flap, the rate of complications after CP was 58.3% (14/24), almost all of which consisted of BFR. The rate of complications in the 3D titanium mesh group was also high, reaching 55%. However, a greater variety of complications was observed than in patients who underwent CP with an autologous bone flap. In the risk factor analysis, we failed to find any factors significantly related with BFR in patients treated with CP using an autologous bone flap, while in the 3D titanium mesh group, only the existence of a V-P shunt system was found to have a statistically significant association with the occurrence of complications.
- BFR, which is well known to be a unique complication of autologous bone flap surgery, occurs as a result of dysregulation of osteoconduction [15]. A few previous studies reported its occurrence rate to be as high as 34.2% [1,3,16,17]. Despite its high frequency, it is difficult to identify significant predisposing factors for BFR because CP is usually the final step after various neurocritical procedures [1]. However, some studies have succeeded in finding predictors of BFR; for example, Kim et al. [1,10] reported that the existence of a shunting system and the DC-CP interval were associated with the risk of BFR after CP, and they also demonstrated that, in order to reduce the rate of BFR, early CP (within 45 days) should be recommended. In contrast, Schuss et al. reported that the presence of multiple fractures, wound healing disturbances, and abscess formation after CP were risk factors for BFR after CP [2]. In another study, a primary synthetic implant, the DC-CP interval, and younger age showed associations with BFR. In our study, we were only able to observe a marginally significant trend for larger bone flaps to be associated with BFR, which may be because larger bone flaps have a higher chance of experiencing dysregulated osteoconduction than small bone flaps [17–19].
- Titanium mesh plates have been introduced as an alternative material for autologous bone flap, with advantages including a low risk of tissue reaction and biological inertness, especially in patients with large bone defects [6]. However, in this study, the risk of minor complications—including mainly cosmetic problems, such as extrusion, dehiscence, and exposure of the titanium mesh—was relatively high, reaching 55%. A V-P shunt system was the only significant risk factor for these overall complications after CP with a 3D titanium mesh. Although the exact mechanism has not been proven yet, we assume that the negative intracranial pressure caused by a V-P shunt system plays a critical role in depressing the patient’s skin and pulling the skin flap into the mesh print, making the mesh print of the implanted titanium visible through the thinned skin. Supporting our hypothesis, Liao and Kao [20] also proposed a similar mechanism for explaining sunken brain after DC. Hence, physicians should consider using other materials without a mesh print, especially in patients who already have a V-P shunt system or who may need shunting after CP.
- There are some limitations of this study. The small sample size with a relatively short-term follow-up period and missing values due to loss to follow-up are important limitations in drawing generalizable conclusions. Furthermore, our study had a retrospective design and enrolled patients at a single center. Hence, selection bias was not avoided.
DISCUSSION
- A relatively high complication rate was observed after CP for surgical bone defects after DC for severe TBI. Different complications occurred depending on the material that was used. In order to avoid BFR after CP with an autologous bone flap, another artificial material may be a better option in patients with a larger surgical bone defect. Furthermore, in order to reduce the rate of complications after CP with a titanium mesh plate, other artificial materials— especially without a mesh print—should be recommended, particularly in patients with a previous V-P shunt system or who may need a shunting system later.
CONCLUSION
ACKNOWLEDGEMENTS
Fig. 1Flow chart showing the inclusion criteria for this study. TBI: traumatic brain injury, PMMA: poly methacrylate, PEEK: polyther ether ketone.


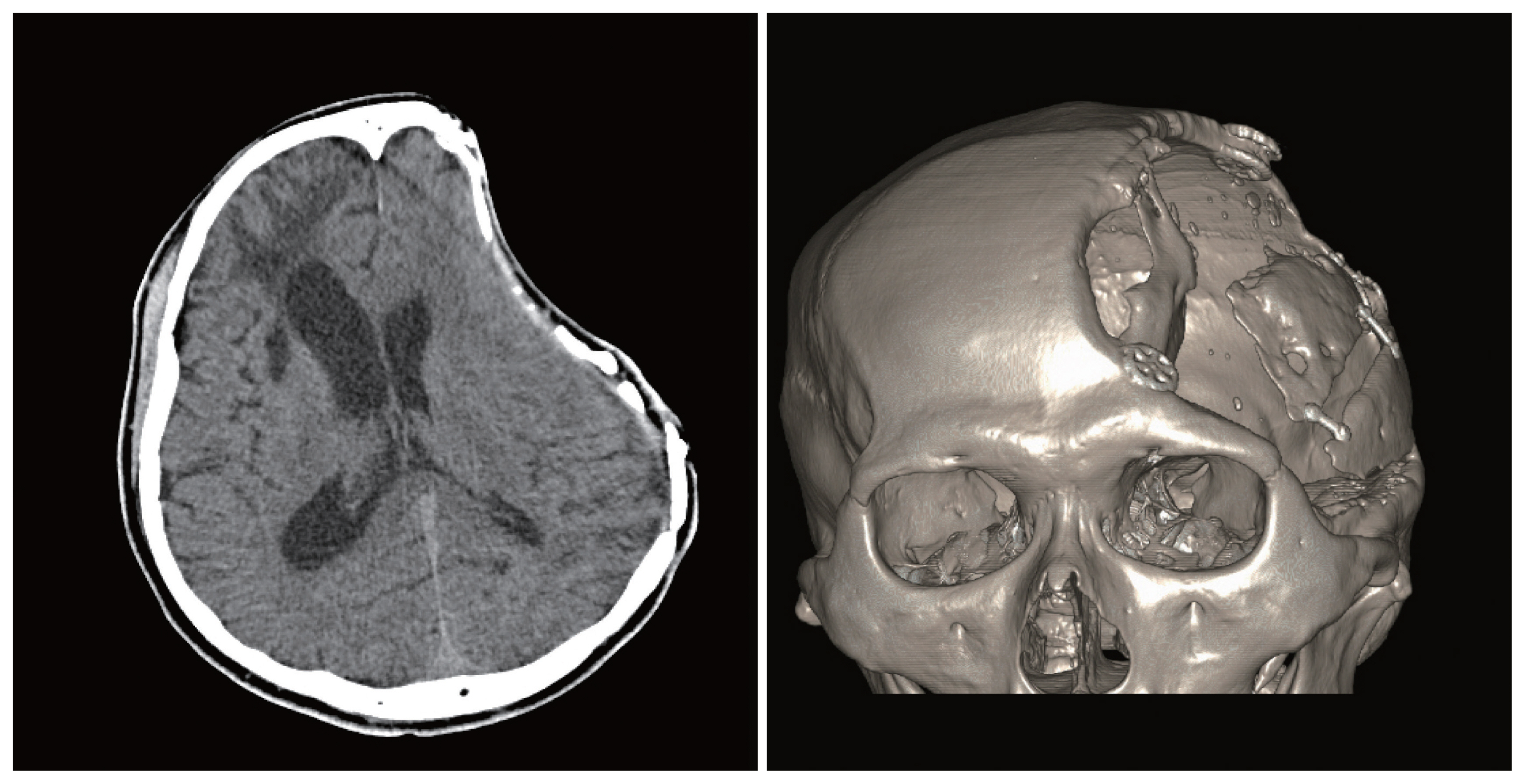
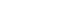
Fig. 2Bone flap resorption on brain computed tomography, in axial and three-dimensional reconstructions.
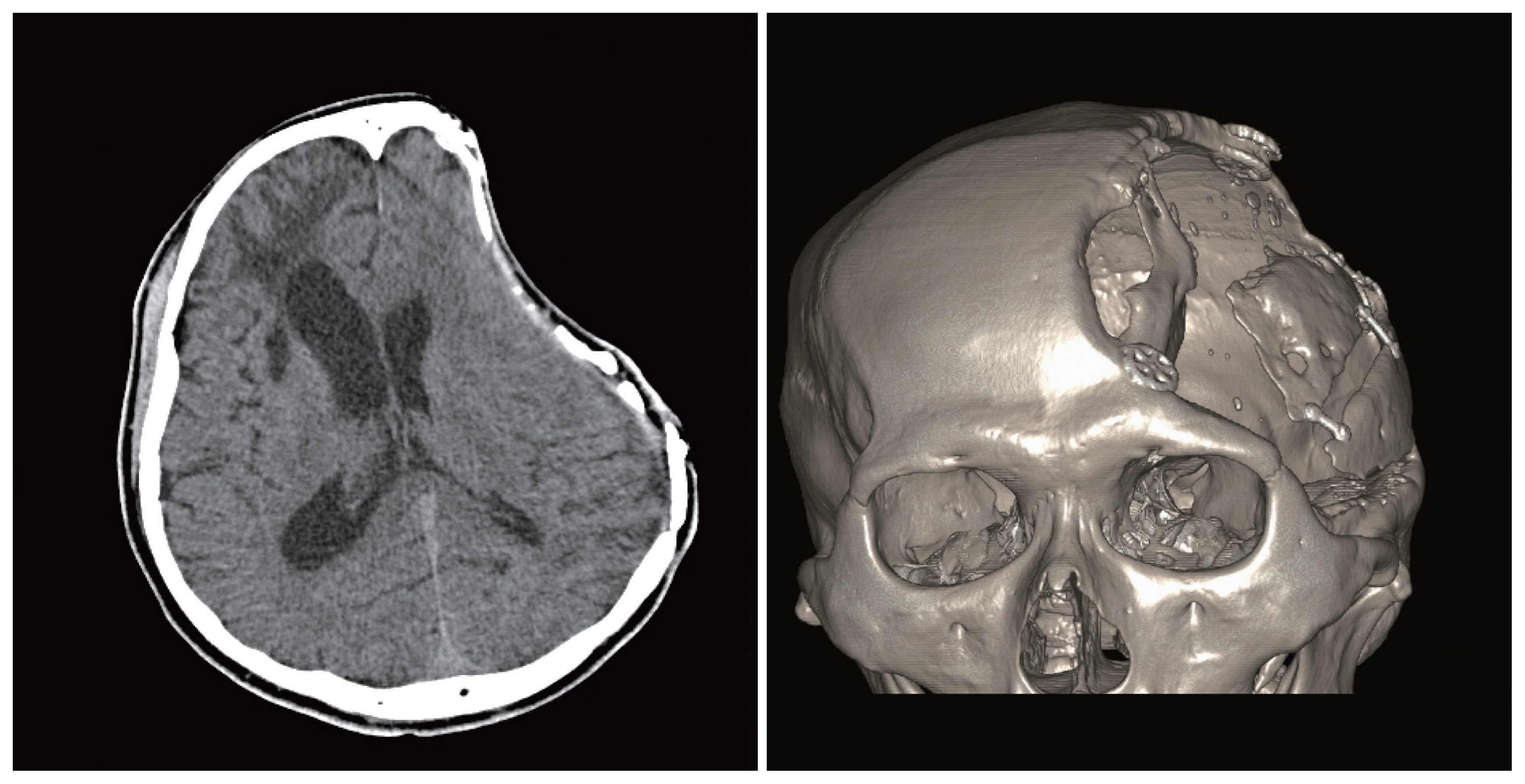

Fig. 3Extrusion after cranioplasty. Extrusion of a mesh was defined as severe thinning of the soft tissue and skin to show the implanted mesh patterns without wound dehiscence. The figures are displayed with permission from the patient.

Table 1Clinical demographics and statistical analysis using the independent t-test or the Fisher’s exact test
| Autologous bone (n=24) | 3D titanium (n=20) | p-value | |
|---|---|---|---|
| Age (years) | 49.0±17.3 | 53.7±11.5 | 0.311 |
| Sex, female (%) | 6:25 | 10:50 | 0.090 |
| V-P shunt (%) | 10:41.7 | 10:50 | 0.913 |
| Flap size (cm2) | 104.2±27.1 | 122.6±34.8 | 0.055 |
| Operation time (ninutes) | 196.7±75.6 | 170.4±128.3 | 0.402 |
| DC-CP interval (days) | 66.3±42.2 | 253.5±351.7 | 0.013a |
- 1. Kim JH, Kim JH, Kwon TH, Chong K, Hwang SY, Yoon WK. Aseptic bone flap resorption after cranioplasty with autologous bone: incidence, risk factors, and clinical implications. World Neurosurg 2018;115:e111–8.ArticlePubMed
- 2. Schuss P, Vatter H, Oszvald A, Marquardt G, Imöhl L, Seifert V, et al. Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma 2013;30:91–5.ArticlePubMed
- 3. Honeybul S, Morrison DA, Ho KM, Lind CR, Geelhoed E. A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J Neurosurg 2017;126:81–90.ArticlePubMed
- 4. Mukherjee S, Thakur B, Haq I, Hettige S, Martin AJ. Complications of titanium cranioplasty--a retrospective analysis of 174 patients. Acta Neurochir (Wien) 2014;156:989–98; discussion 998, ArticlePubMedPDF
- 5. Lai JB, Sittitavornwong S, Waite PD. Computer-assisted designed and computer-assisted manufactured polyetheretherketone prosthesis for complex fronto-orbito-temporal defect. J Oral Maxillofac Surg 2011;69:1175–80.ArticlePubMed
- 6. Roh H, Kim J, Kim JH, Chong K, Yoon WK, Kwon TH, et al. Analysis of complications after cranioplasty with a customized three-dimensional titanium mesh plate. World Neurosurg 2019;123:e39–44.ArticlePubMed
- 7. Thien A, King NK, Ang BT, Wang E, Ng I. Comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg 2015;83:176–80.ArticlePubMed
- 8. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res 1997;19:311–6.ArticlePubMed
- 9. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg 2010;112:1120–4.ArticlePubMed
- 10. Kim JH, Hwang SY, Kwon TH, Chong K, Yoon WK, Kim JH. Defining “early” cranioplasty to achieve lower complication rates of bone flap failure: resorption and infection. Acta Neurochir (Wien) 2019;161:25–31.ArticlePubMedPDF
- 11. Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT. A retrospective analysis and review of an institution’s experience with the complications of cranioplasty. Br J Neurosurg 2013;27:629–35.ArticlePubMed
- 12. Hill CS, Luoma AM, Wilson SR, Kitchen N. Titanium cranioplasty and the prediction of complications. Br J Neurosurg 2012;26:832–7.ArticlePubMed
- 13. Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S. Cranioplasty with custom-made titanium plates--14 years experience. Neurosurgery 2013;72:248–56; discussion 256, ArticlePubMedPDF
- 14. Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus 2009;26:E10, Article
- 15. Kalfas IH. Principles of bone healing. Neurosurg Focus 2001;10:E1, Article
- 16. Ewald C, Duenisch P, Walter J, Götz T, Witte OW, Kalff R, et al. Bone flap necrosis after decompressive hemicraniectomy for malignant middle cerebral artery infarction. Neurocrit Care 2014;20:91–7.ArticlePubMedPDF
- 17. Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med 2015;23:75, ArticlePubMedPMC
- 18. Kriegel RJ, Schaller C, Clusmann H. Cranioplasty for large skull defects with PMMA (polymethylmethacrylate) or tutoplast processed autogenic bone grafts. Zentralbl Neurochir 2007;68:182–9.ArticlePubMedPDF
- 19. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg 2004;100(2 Suppl Pediatrics):163–8.ArticlePubMed
- 20. Liao CC, Kao MC. Cranioplasty for patients with severe depressed skull bone defect after cerebrospinal fluid shunting. J Clin Neurosci 2002;9:553–5.ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Customized Additive Manufacturing in Bone Scaffolds—The Gateway to Precise Bone Defect Treatment
Juncen Zhou, Carmine Wang See, Sai Sreenivasamurthy, Donghui Zhu
Research.2023;[Epub] CrossRef - Customized cost-effective polymethylmethacrylate cranioplasty: a cosmetic comparison with other low-cost methods of cranioplasty
Manish Baldia, Mathew Joseph, Suryaprakash Sharma, Deva Kumar, Ashwin Retnam, Santosh Koshy, Reka Karuppusami
Acta Neurochirurgica.2022; 164(3): 655. CrossRef - Letter to the Editor: Complications following titanium cranioplasty compared with nontitanium implants cranioplasty: A systematic review and meta-analysis
Michael Amoo, Jack Henry
Journal of Clinical Neuroscience.2021; 87: 32. CrossRef - Complications of Cranioplasty in Relation to Material: Systematic Review, Network Meta-Analysis and Meta-Regression
Jack Henry, Michael Amoo, Joseph Taylor, David P O’Brien
Neurosurgery.2021; 89(3): 383. CrossRef - Comparison of complications in cranioplasty with various materials: a systematic review and meta-analysis
Liming Liu, Shou-Tao Lu, Ai-Hua Liu, Wen-Bo Hou, Wen-Rui Cao, Chao Zhou, Yu-Xia Yin, Kun-Shan Yuan, Han-Jie Liu, Ming-Guang Zhang, Hai-Jun Zhang
British Journal of Neurosurgery.2020; 34(4): 388. CrossRef - A Reappraisal of the Necessity of a Ventriculoperitoneal Shunt After Decompressive Craniectomy in Traumatic Brain Injury
Seunghan Yu, Hyuk Jin Choi, Jung Hwan Lee, Mahnjeong Ha, Byung Chul Kim
Journal of Trauma and Injury.2020; 33(4): 236. CrossRef
 KST
KST

 PubReader
PubReader ePub Link
ePub Link Cite
Cite